Hepatitis B
About Hepatitis B Virus (HBV)
The WHO estimates that 257 million people were living with chronic hepatitis B infection in 2015. Chronic HBV can lead to cirrhosis (scarring of the liver), liver failure or liver cancer (hepatocellular carcinoma). In 2015, hepatitis B resulted in an estimated 887 000 deaths, mostly from cirrhosis and hepatocellular carcinoma (i.e. primary liver cancer).
Without a scale-up of effective interventions, it is estimated that HBV will lead to 11.8 million deaths by 2030. Although high alcohol consumption is one of the main causes of cirrhosis in Europe and North America, in many Asian and African countries HBV is the most common cause of chronic liver disease and mainly affects young people. In 2016, the United Nations General Assembly and the World Health Organization (WHO) incorporated viral hepatitis elimination in the 2030 agenda for sustainable development. Africa, which accounts for about 80 million HBV-infected people at risk of serious hepatic complications, has been identified as a priority region for research on hepatitis B due to the high burden of hepatitis B and the absence of guidelines adapted to the local setting.
PROLIFICA HBV Research
The PROLIFICA programme started in November 2011 in The Gambia and Senegal where the team undertook the West Africa (WATCH-B study). The study was supported by Imperial College London, the Medical Research Council Gambia (MRCG) Unit at the London School of Hygiene and Tropical Medicine, The University Cheikh Anta Diop in Dakar, INSERM France and the International Agency for Research on Cancer (IARC).
Between December 2011 and January 2014, the PROLIFICA team screened approximately 16,000 subjects for HBV in Gambia and Senegal (prevalence around 10%). Of these subjects, 2,108 were HBV-infected patients, with 1,192 patients in The Gambia and 916 in Senegal. These HBV-infected patients had a comprehensive liver assessment using high quality reference tests (e.g HBV DNA, liver biopsy or Fibroscan). To date, 283 patients have been treated with Tenofovir Disoproxil Fumarate (TDF) for more than 5 years. Treatment is provided by the US company, Gilead. A total of 1,825 patients have not been treated since they did not fulfill the current international treatment criteria.
PROLIFICA is a unique research platform in Sub-Saharan Africa. It has generated high quality data that has been used by the WHO to develop guidelines and reports on viral hepatitis B in Africa. From the WATCH-B study, PROLIFICA has generated two cohorts of patients with chronic hepatitis B infection. We continue to follow these patients to assess the rate of liver disease progression in HBV-infected individuals exposed to the African environment. The follow-up study is known as the MATCH-B study.
PROLIFICA HBV research activities focus on four main objectives:
- Demonstrate that screening for chronic hepatitis B and treatment of the infection is an effective way of reducing the burden of liver cancer in Africa;
- Develop simplified diagnostic and monitoring algorithms to scale-up screen-and-treat interventions and eventually eliminate HBV in Africa;
- Assess the rate and risk factors of liver disease progression in the African environment;
- Identify strategies adapted to the African setting to prevent early transmission of the virus.
You can find our more information about our HBV studies in Africa via the following links:
WATCH-B
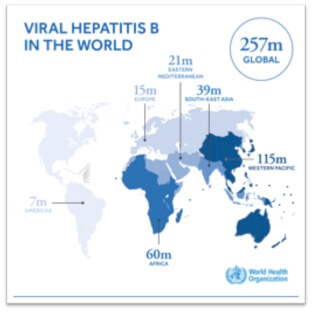 Source: WHO, 2017
Source: WHO, 2017
